SciTechDaily
Innovation & Investment
S
S
The NAs. Home/
Scientists Watched Human Embryos Implant for the First Time
An artificial womb allowed scientists to watch human and mouse embryos implant in real time, providing a way to better understand fertility and pregnancy loss.
Written by Andrea Lius, Ph.D
Aug 16, 2025| 3 min read
Only about one out of three conceptions leads to birth.1 Approximately a third of embryos fail to attach to the uterus, while another third is lost after the embryo implants.
“Human reproduction is quite inefficient, and we can consider implantation [as] the bottleneck,” said Amélie Godeau, a biophysicist at the Institute for Bioengineering of Catalonia, in a press release video.
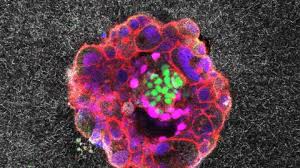
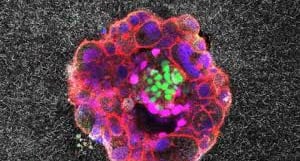
Researchers didn’t have a good way to study this process in the lab—until now. Recently, Godeau and her team developed a synthetic platform to model human embryonic development during implantation and beyond.2 Their work, published in Science Advances, allowed the researchers to visualize human embryo implantation live for the first time. This advance may one day help solve issues associated with fertility and pregnancy losses.
Godeau's team developed two systems, one 2D and the other 3D, to mimic different stages of embryo implantation. To simulate the extracellular environment that human embryos encounter while attaching to the womb, both platforms consisted of collagen, which is abundant in the uterus, and other proteins that are critical in early development. The researchers ensured that implanted embryos were developing properly in their synthetic womb systems by staining for standard marker proteins such as OCT4, GATA6, and CK7.
Using a high-resolution microscope, the team made time-lapse movies which showed the embryo's interaction with the synthetic matrix around it. The researchers also quantified how the embryo displaced its surrounding matrix, capturing the biomechanical dynamics of this contact.
NEWS
The Nobel PrizeThe Nobel Prize
BREAKING NEWS
The Nobel Assembly at the Karolinska Institutet has today decided to award the 2024 Nobel Prize in Physiology or Medicine to Victor Ambros and Gary Ruvkun for the discovery of microRNA and its role in post-transcriptional gene regulation.
This year’s Nobel Prize honours two scientists for their discovery of a fundamental principle governing how gene activity is regulated.
The information stored within our chromosomes can be likened to an instruction manual for all cells in our body. Every cell contains the same chromosomes, so every cell contains exactly the same set of genes and exactly the same set of instructions. Yet, different cell types, such as muscle and nerve cells, have very distinct characteristics. How do these differences arise? The answer lies in gene regulation, which allows each cell to select only the relevant instructions. This ensures that only the correct set of genes is active in each cell type.
Their groundbreaking discovery revealed a completely new principle of gene regulation that turned out to be essential for multicellular organisms, including humans. It is now known that the human genome codes for over one thousand microRNAs. Their surprising discovery revealed an entirely new dimension to gene regulation. MicroRNAs are proving to be fundamentally important for how organisms develop and function.
Cell therapy weekly: US$115 million secured for bioprinted tissue therapies
16 Jan 2025
Written by Megan Giboney
This week: Aspen Neuroscience (CA, USA) and Mytos (London, UK) entered a manufacturing collaboration for Aspen’s investigational cell therapy for Parkinson’s disease, CTMC (TX, USA) partnered with Syenex (IL, USA) to improve the efficiency and scalability of engineered T-cell therapies and Aspect Biosystems (Vancouver, Canada) raised US$115 million to advance its bioprinted tissue therapeutics toward clinical trials.
https://www.regmednet.com/cell-therapy-weekly-us115-million-secured-for-bioprinted-tissue-therapies/




Cell NEWS
04 February 2025
The science behind the first pig-organ transplant trial in humans
The small trial will help to establish whether kidneys from genetically modified pigs can be transplanted into people safely and effectively.
The first clinical trial testing whether pig kidneys can be safely transplanted into living people has been approved by the US Food and Drug Administration (FDA). As part of the trial, which will begin later this year, kidneys from genetically modified pigs will be transplanted into people with chronic kidney disease whose organs no longer function independently.
The FDA’s green light will bring the experimental procedure a step closer to one day supplying organs to the thousands of people who are waiting for a donor organ. “The start of formal clinical trials is very exciting,” says Jay Fishman, a specialist in transplant infectious diseases at Massachusetts General Hospital in Boston.
About half a dozen people in the United States and China have received organs from genome-edited pigs — kidneys, hearts, liver and a thymus — but the surgeries were approved on compassionate grounds, meaning the patients were very sick and had no other options. Most recipients did not survive beyond a few months after the transplants for various reasons, including that they were too sick to cope with major surgery.
Scientific Excellence
ells/research-on-iss-to-determine-if-stem-cell-production-in-space-can-improve-cancer-treatments-on-earth/?MailingID=%DEPLOYMENTID%&utm_medium=newsletter&utm_source=Inside+Precision+Medicine+Today&utm_content=01&utm_campaign=Inside+Precision+Medicine+Today_20230802
August 2, 2023
Research on ISS to Determine If Stem Cell Production in Space Can Improve Cancer Treatments on Earth
Just after 8:30 ET on Tuesday night a Commercial Resupply Services mission to the International Space Station (ISS) launched carrying 20 payloads of a diverse set of research projects to determine the benefits of working in microgravity. One of the projects, designed by Mayo Clinic and ClinImmune scientists in collaboration with space technology platform companies Sierra Space and BioServe Space Technologies, will conduct research on the ISS to determine if stem cell production in space can improve cancer treatments for patients on earth.
https://www.insideprecisionmedicine.com/topics/precision-medicine-topic/stem-c
https://sbr2024.org/
SWINE IN BIOMEDICAL RESEARCH CONFERENCE 2024
June 14 – 18, 2024 | Madison, WI, USA
The Swine in Biomedical Research Conference 2024 (SBR 2024), hosted at the lakeside University of Wisconsin-Madison, is the eighth in the quintessential conference series. Since its inception in 1995, advances from the sequencing of the pig genome to the recent evolution of gene editing technologies have dramatically changed the landscape of porcine models. SBR 2022 welcomed a new era of translational research in pigs, that promises to accelerate the discovery and development of clinically relevant therapies and technologies. SBR 2024 will advance this mission and continue to foster collaborations between clinicians, biomedical researchers and animal scientists, and spark partnerships with the biomedical industry.
The planned sessions will:Identify unmet clinical needs and existing gaps in research.Showcase porcine models and reveal their strengths and limitations.Highlight innovative use of models for tackling clinical problems.Discuss novel tools for advancing biomedical swine research.Explore the need for biomedical swine research facilities, resources and expertise (Special Panel Session).
New Japanese stem cell treatment raises hopes, and ethical questions
https://insightplus.mja.com.au › a-new-japanese-stem-cell...
The Japanese health ministry approved Stemirac last December, and the treatment is now available to the Japanese public, with most of the $140,000 cost covered by the country's universal National Health Insurance.
So far, only three treatments have earned conditional approval, but the government aims to add to the list. Japan’s economic development ministry has estimated that regenerative medicine will be a $10 billion market in the country by 2030.

Stem Cell Storage Partnership Between LMRUK and Smart Cells International Can be a Life Saver
By Biobanking.com
Leukaemia & Myeloma Research UK (LMRUK) and Smart Cells International, the UK’s first private stem cell storage company, have collaborated to procure, process and preserve cord blood stem cells using LMRUK’s cord blood banking facility. The Model Cell Biobank, established in 2015 by LMRUK, provides eligible families with the opportunity to preserve their newborn’s umbilical […]
Historic deal for Henrietta Lacks’s family
The family of Henrietta Lacks and the biotech company Thermo Fisher Scientific have reached a confidential settlement over the unethical use of Lacks’s cells.
The company profited from products containing a cell line that was started in 1951 using cancer tissue taken from Lacks and used without her consent. The cell line has been instrumental in many scientific discoveries and continues to be widely used, but the family was never compensated. “It does open up a conversation about needing to know, where do things come from initially, and what if terrible and awful, horrific things happen?” says legal specialist Caprice Roberts. The Lacks family’s lawyers indicated that similar lawsuits might follow.


www.pasteur.fr
Feb 20, 2023 · The IciStem consortium, of which the Institut Pasteur is a partner, has described the third case of probable cure of HIV infection in the world. The person concerned is a man
Nature Medicine